Chemical bonds are fundamental to understanding the interactions between atoms and molecules. They determine the properties and behaviors of matter. Here, we explore the main types of chemical bonds: ionic, covalent, and metallic.
Ionic Bonds: Electrons Exchange to Form Stability
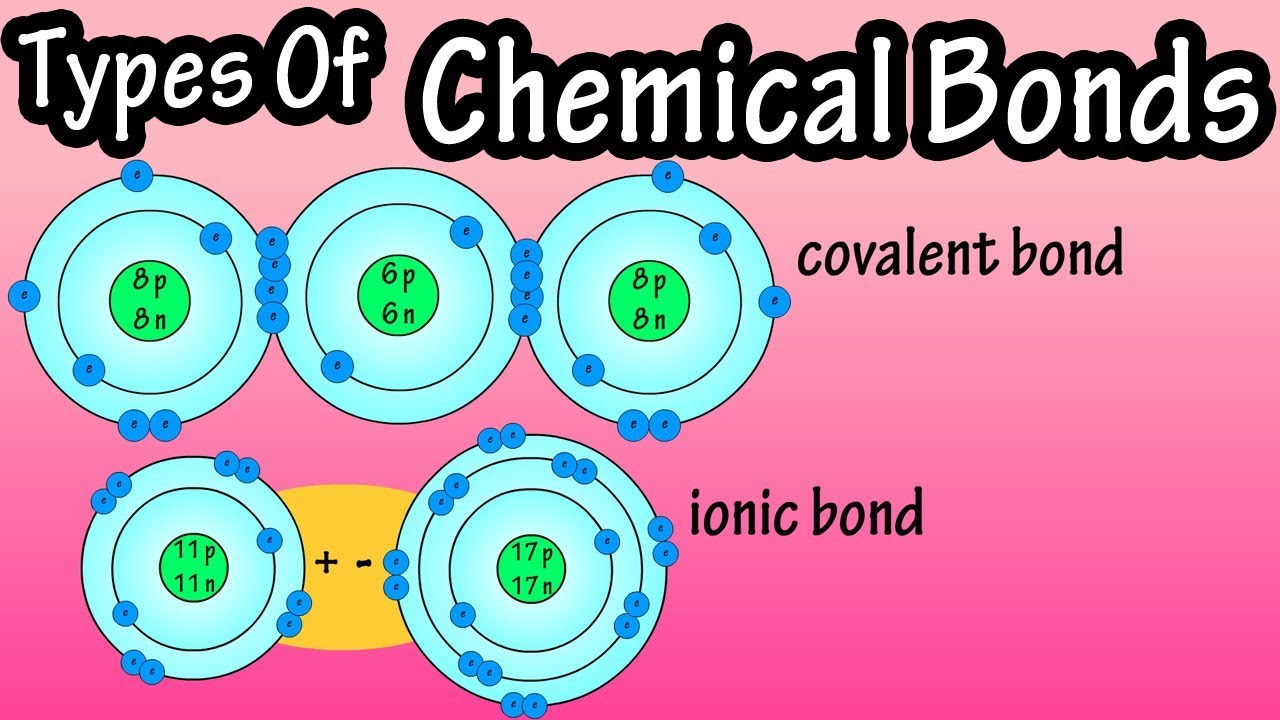
Ionic bonds form between atoms when one atom transfers electrons to another, resulting in the formation of ions—positively and negatively charged atoms or molecules. These bonds occur typically between elements with a large difference in electronegativity, such as sodium (Na) and chlorine (Cl) in sodium chloride (NaCl).
Ionic bonds are characterized by the complete transfer of valence electrons from one atom to another, leading to the formation of an anion (negative ion) and a cation (positive ion). The electrostatic attraction between these oppositely charged ions creates a strong bond.

Types of Chemical Bonds Explained
Covalent Bonds: Shared Electrons Create Stability
Covalent bonds occur when atoms share one or more pairs of electrons to achieve a stable electron configuration. This type of bonding is common among non-metallic elements like hydrogen (H2), oxygen (O2), and carbon (C) in organic molecules.
Covalent bonds can be further classified into single, double, or triple bonds based on the number of electron pairs shared between atoms. These bonds are strong and directional, influencing molecular shapes and properties significantly.
Metallic Bonds: Delocalized Electrons Create Conductivity
Metallic bonds are unique to metals and alloys. In metallic bonding, atoms within a metal lattice donate their electrons freely to create a “sea” of delocalized electrons. This electron cloud allows metals to conduct electricity and heat efficiently.
The strength of metallic bonds contributes to the high melting and boiling points observed in metals. These bonds also give metals their characteristic luster and malleability, as atoms can slide past each other without breaking the bond.
Transition Words and Active Voice Enhance Clarity
Transition words like “however,” “moreover,” and “therefore” help to connect ideas smoothly. Using active voice makes the writing clearer and more engaging. For example, “Ionic bonds form when…” instead of “Ionic bonds are formed when…”