The chemistry of gases involves understanding how gases behave under various conditions and how they react with other substances. This field is critical for applications ranging from industrial processes to environmental science. This article provides a detailed overview of gas behavior, fundamental gas laws, and common reactions involving gases.
Chemistry of Gases: Behavior and Reactions
Fundamental Properties of Gases
Pressure
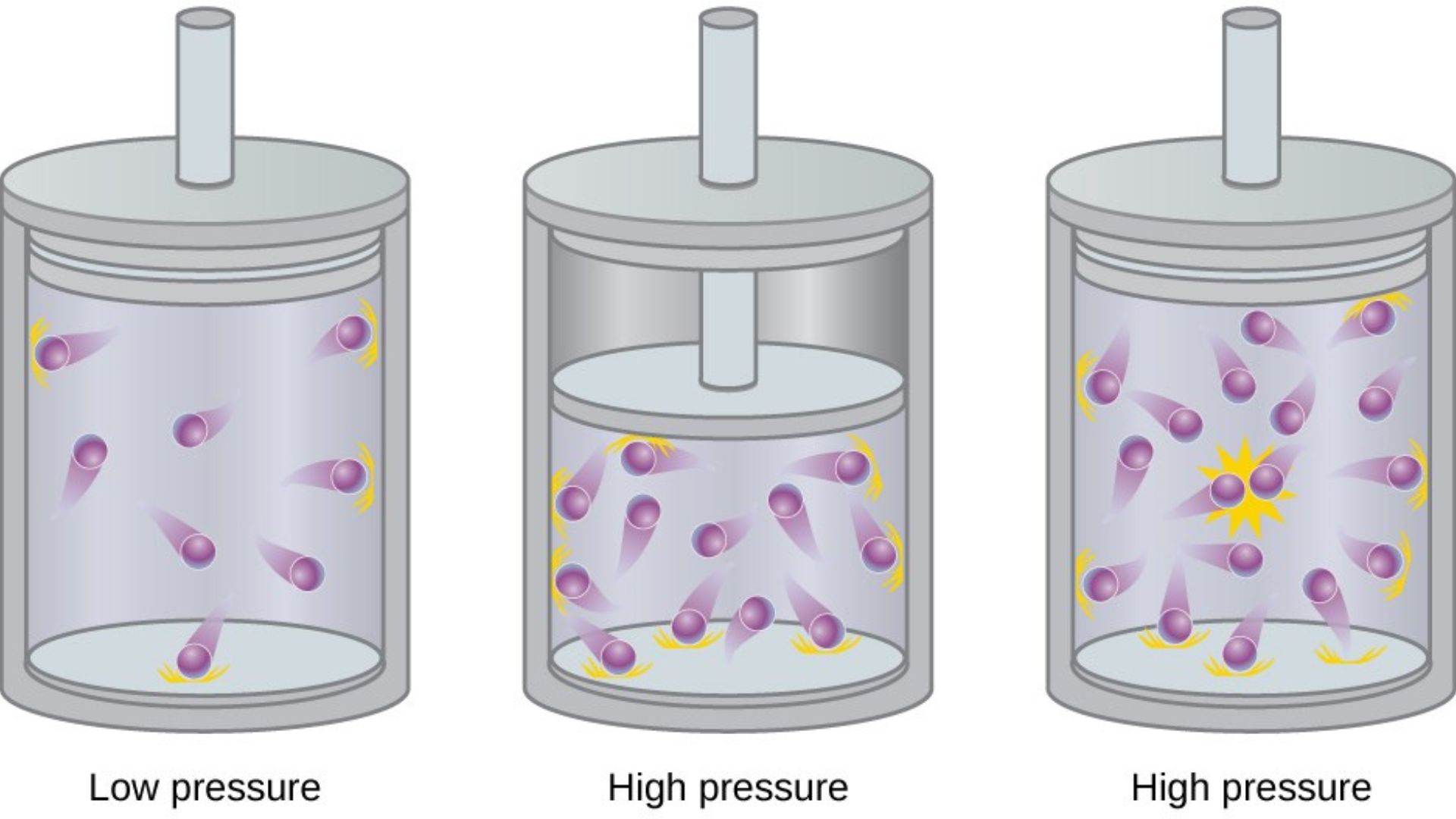
Pressure is the force exerted by gas molecules on the walls of their container. It is measured in units such as atmospheres (atm), Pascals (Pa), or millimeters of mercury (mm Hg). The pressure of a gas is influenced by its volume, temperature, and the number of molecules present.
Volume
Volume is the amount of space that a gas occupies. It is typically measured in liters (L) or cubic meters (m³). The volume of a gas is directly related to its pressure and temperature, as described by gas laws.
Temperature
Temperature is a measure of the average kinetic energy of gas molecules. It is usually measured in degrees Celsius (°C) or Kelvin (K). Temperature affects the speed of gas molecules and their interactions, influencing properties such as pressure and volume.
Molecular Weight
The molecular weight of a gas is the mass of one mole of its molecules, measured in grams per mole (g/mol). It plays a role in determining the density of the gas and its behavior in reactions.
Gas Laws
Boyle’s Law
Boyle’s Law states that the pressure of a gas is inversely proportional to its volume when temperature is constant. Mathematically, P×V=constantP \times V = \text{constant}. This means that if the volume of a gas decreases, its pressure increases, and vice versa.
Charles’s Law
Charles’s Law states that the volume of a gas is directly proportional to its temperature when pressure is constant. Mathematically, VT=constant\frac{V}{T} = \text{constant}. As the temperature of a gas increases, its volume also increases, provided the pressure remains unchanged.
Avogadro’s Law
Avogadro’s Law states that the volume of a gas is directly proportional to the number of moles of gas when pressure and temperature are constant. Mathematically, Vn=constant\frac{V}{n} = \text{constant}. This implies that equal volumes of gases, at the same temperature and pressure, contain the same number of molecules.
Ideal Gas Law
The Ideal Gas Law combines Boyle’s, Charles’s, and Avogadro’s laws into a single equation: PV=nRTPV = nRT. Here, PP is pressure, VV is volume, nn is the number of moles of gas, RR is the gas constant, and TT is temperature. This law provides a comprehensive description of gas behavior under ideal conditions.
Real Gas Behavior
Real gases deviate from ideal behavior at high pressures and low temperatures. The Van der Waals equation corrects for these deviations by accounting for intermolecular forces and the volume occupied by gas molecules.
Gas Reactions
Combustion Reactions
Combustion is a chemical reaction between a fuel and an oxidant, usually oxygen, that produces heat and light. Incomplete combustion can produce carbon monoxide and other byproducts. For example, the combustion of methane can be represented by the equation:
CH4+2O2→CO2+2H2O\text{CH}_4 + 2\text{O}_2 \rightarrow \text{CO}_2 + 2\text{H}_2\text{O}
Synthesis Reactions
In synthesis reactions, two or more reactants combine to form a single product. For example, the reaction between hydrogen and nitrogen to form ammonia is a synthesis reaction:
3H2+N2→2NH33\text{H}_2 + \text{N}_2 \rightarrow 2\text{NH}_3
Decomposition Reactions
Decomposition reactions involve a single compound breaking down into two or more products. For example, the decomposition of water into hydrogen and oxygen gases can be represented by:
2H2O→2H2+O22\text{H}_2\text{O} \rightarrow 2\text{H}_2 + \text{O}_2
Displacement Reactions
Displacement reactions occur when one element replaces another in a compound. For example, when hydrochloric acid reacts with zinc, zinc displaces hydrogen:
Zn+2HCl→ZnCl2+H2\text{Zn} + 2\text{HCl} \rightarrow \text{ZnCl}_2 + \text{H}_2
Applications and Implications
Industrial Applications
Understanding gas behavior and reactions is crucial in various industries, including chemical manufacturing, energy production, and environmental management. For example, controlling the conditions of gas reactions is essential for optimizing the production of chemicals and fuels.
Environmental Impact
Gases play a significant role in environmental processes, such as the greenhouse effect and air pollution. Understanding how gases interact and react can help in developing strategies to mitigate environmental impacts and address climate change.
Safety Considerations
Handling gases requires careful attention to safety, including proper storage, ventilation, and monitoring of gas concentrations. Understanding the properties and reactions of gases helps in preventing accidents and ensuring safe working conditions.
Conclusion
The chemistry of gases encompasses the study of their properties, behavior, and reactions. Understanding gas laws, the nature of gas reactions, and their applications is essential for both practical and scientific purposes. By applying these principles, researchers and professionals can better manage and utilize gases in various contexts.