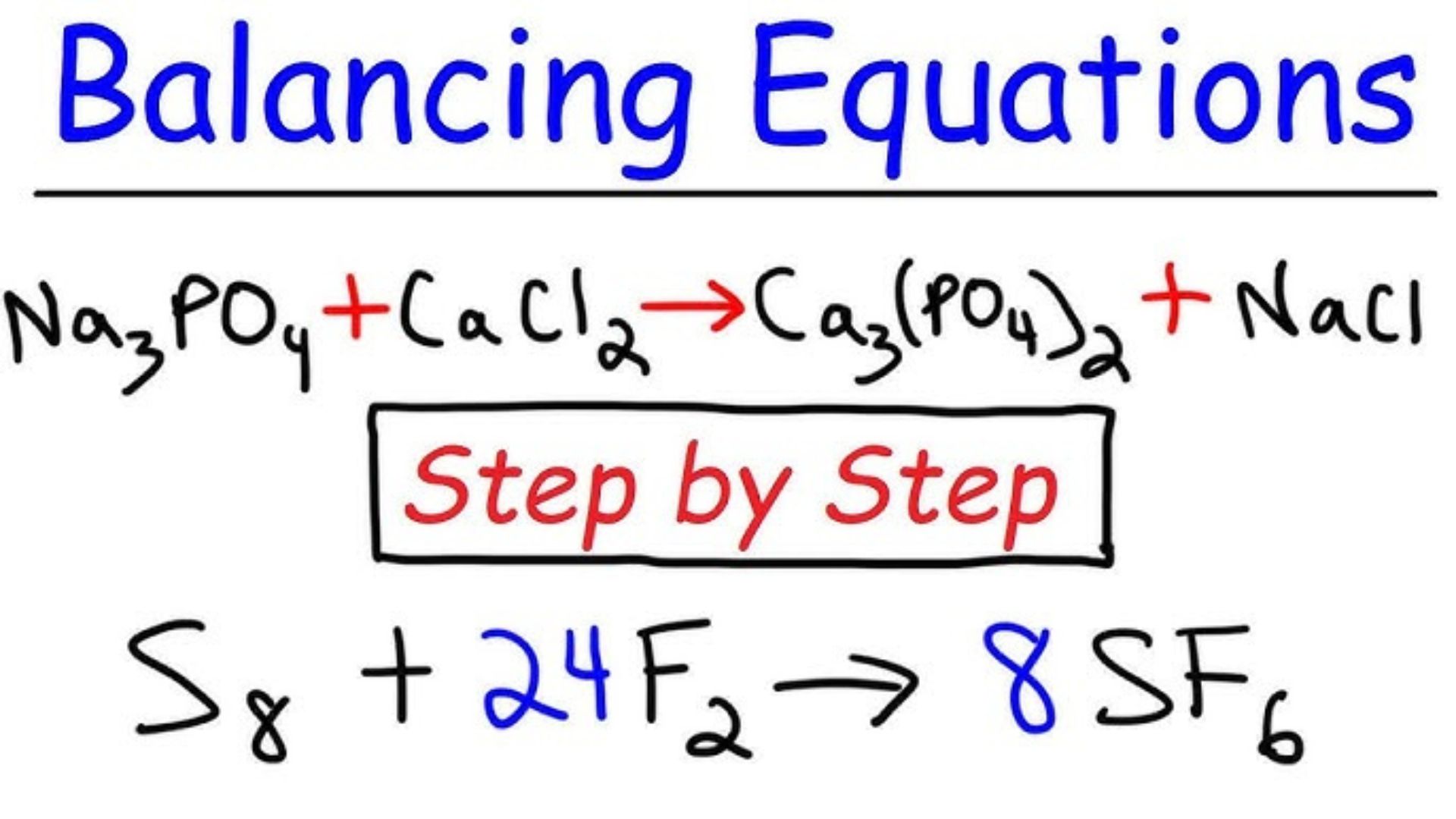
Without a balanced equation, it’s impossible to understand the proportions of reactants and products involved. Here, we’ll outline the best techniques for balancing chemical equations effectively.
Understanding the Basics
Before going into the techniques, it’s crucial to understand the basics of balancing chemical equations. A balanced chemical equation has the same number of atoms for each element on both the reactant and product sides.

Balancing Chemical Equations
Counting Atoms
The first step in balancing chemical equations is counting the number of atoms for each element in the reactants and products. Write down these counts and ensure you know which elements need balancing. This step provides a clear picture of the imbalance and where adjustments are necessary.
Balancing One Element at a Time
One effective technique for balancing chemical equations is to balance one element at a time. Start with the element that appears in the least number of compounds and balance it across the equation. Move on to the next element and repeat the process. This method simplifies the balancing process by breaking it down into manageable steps.
Using Coefficients
Adjusting the coefficients (the numbers in front of compounds) is essential in balancing chemical equations. Changing coefficients alters the number of molecules or moles involved without changing the identity of the compounds. Always start with whole numbers and adjust them as needed to balance the equation.
Balancing Polyatomic Ions as a Unit
When dealing with polyatomic ions (ions composed of multiple atoms), it can be helpful to balance them as a single unit if they appear unchanged on both sides of the equation. This approach simplifies the process and reduces the number of adjustments needed.
Balancing Hydrogen and Oxygen Last
In many reactions, especially combustion reactions, it’s easiest to balance hydrogen and oxygen atoms last. These elements often appear in multiple compounds, so balancing them first can complicate the process.
Using the Algebraic Method
For more complex equations, the algebraic method can be useful. This technique involves assigning variables to each coefficient and writing algebraic equations based on the number of atoms. Solving these equations simultaneously helps find the correct coefficients to balance the equation.
Checking Your Work
After balancing the chemical equation, always double-check your work. Ensure that the number of atoms for each element is equal on both sides of the equation.
Practicing Regularly
Balancing chemical equations is a skill that improves with practice. Regularly working on different types of equations enhances your understanding and efficiency. Practice not only helps in mastering the techniques but also builds confidence in handling complex reactions.
Using Online Tools and Resources
There are many online tools and resources available to assist with balancing chemical equations. These tools provide step-by-step guidance and instant feedback, making them valuable for learning and practice. Utilizing these resources can reinforce your understanding and improve your skills.
Visualizing the Reaction
Visualizing the reaction can also aid in balancing chemical equations. Drawing diagrams or using models to represent the reactants and products can provide a clearer understanding of the atom counts and how they interact. This visual approach can simplify the balancing process.
Common Mistakes to Avoid
Avoiding common mistakes is crucial in balancing equations. Some common errors include not checking atom counts carefully, changing subscripts instead of coefficients, and ignoring the law of conservation of mass. Being aware of these mistakes helps prevent them and ensures accurate balancing.
Keeping a Balanced Approach
Balancing chemical equations requires a balanced approach—literally and figuratively. Staying patient, methodical, and systematic helps in achieving the correct balance. Rushing through the process often leads to mistakes, so take your time and follow each step carefully.
Seeking Help When Needed
If you encounter difficulties in balancing chemical equations, don’t hesitate to seek help. Consult textbooks, ask teachers, or join study groups. Collaborative learning can provide new insights and make the process easier and more enjoyable.
Conclusion
Balancing chemical equations is a critical skill in chemistry that ensures an accurate representation of chemical reactions. By using these techniques, you can balance equations effectively and understand the stoichiometry of reactions. Regular practice, attention to detail, and a systematic approach are key to mastering this skill.