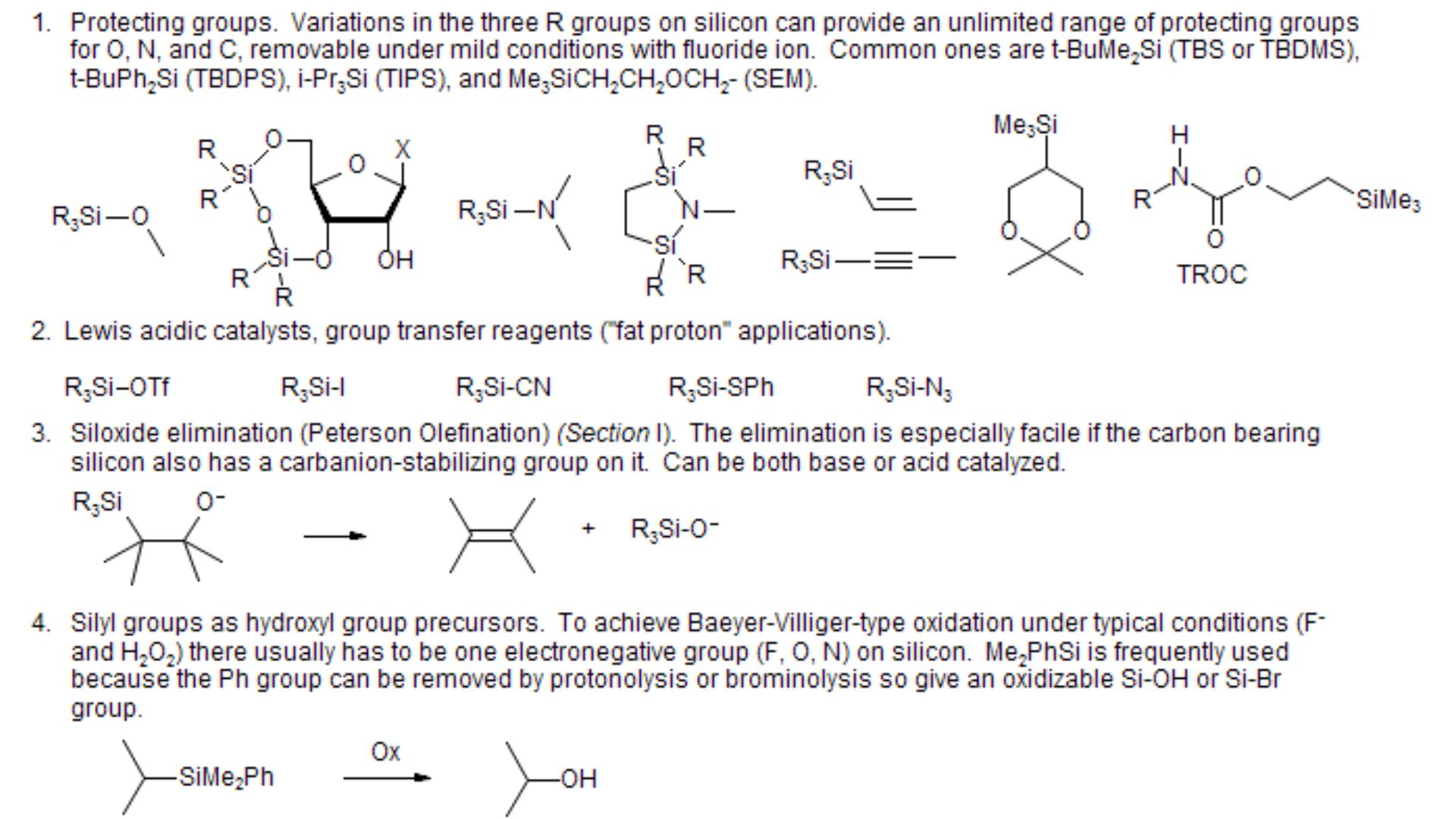
Aldehydes vs ketones are two classes of organic compounds that contain a carbonyl group (C=O), but their structures differ in the placement of this group. In aldehydes, the carbonyl group is bonds to at least one hydrogen atom, making them terminal groups located at the end of a carbon chain. This structural arrangement is denotes as R-CHO, where R can be a hydrogen atom or a hydrocarbon chain. In contrast, ketones have the carbonyl group bonded to two carbon atoms, making them internal groups located within the carbon chain. This structure is represents as R-CO-R’, where R and R’ are hydrocarbon chains. The positioning of the carbonyl group in aldehydes and ketones influences their reactivity and properties.

Aldehydes vs. Ketones: Key Differences
Reactivity and Chemical Behavior
The reactivity of aldehydes and ketones varies due to the differences in their structures. Aldehydes are generally more reactive than ketones. This increases reactivity and is attributed to the hydrogen atom attaches to the carbonyl group in aldehydes, which makes the carbonyl carbon more electrophilic and susceptible to nucleophilic attack. As a result, aldehydes readily participate in oxidation reactions, forming carboxylic acids, and in nucleophilic addition reactions, such as the formation of alcohols. Ketones, on the other hand, are less reactive because the carbonyl carbon bonds to two alkyl groups, which provide electron-donating effects and reduce the electrophilicity of the carbonyl carbon. This makes ketones less prone to oxidation, but they still undergo nucleophilic addition reactions, although at a slower rate compared to aldehydes.
Uses and Applications
Aldehydes and ketones have distinct uses and applications based on their chemical properties. Aldehydes, such as formaldehyde and benzaldehyde, are widely used in the chemical industry. Formaldehyde in the production of plastics, resins, and as a disinfectant, while benzaldehyde is used in the synthesis of perfumes, dyes, and flavoring agents. Ketones, such as acetone and butanone, are important solvents in the pharmaceutical and cosmetic industries. Acetone, in particular, is widely common as a solvent for cleaning and in the production of plastics and synthetic fibers. Both aldehydes and ketones are also essential intermediates in organic synthesis, serving as building blocks for various chemical reactions and the synthesis of more complex molecules.
Biological Significance
In the biological realm, aldehydes and ketones play significant roles. Aldehydes, such as glucose, are important in metabolism and energy production. Glucose, an aldehyde, is a crucial energy source for living organisms and involves glycolysis and the citric acid cycle. Ketones, such as acetone and acetoacetic acid, during the metabolism of fatty acids and are important energy sources during fasting or low-carbohydrate diets. These compounds, known as ketone bodies, provide an alternative energy source for the brain and muscles when glucose levels are low. The presence and balance of aldehydes and ketones in biological systems are essential for maintaining metabolic functions and energy homeostasis.
Identification and Testing
Identifying aldehydes vs ketones can achieve through various chemical tests, each exploiting the unique properties of these compounds. The Tollens’ test is commonly used to distinguish aldehydes from ketones. In this test, an aldehyde reacts with Tollens’ reagent (a solution of silver nitrate in ammonia), producing a silver mirror on the inside of the test tube due to the reduction of silver ions to metallic silver. Ketones do not react with Tollens’ reagent, providing a clear differentiation. Another test is the Fehling’s test, where aldehydes reduce Fehling’s solution (a mixture of copper sulfate, sodium hydroxide, and potassium sodium tartrate) to form a red precipitate of copper(I) oxide. Ketones, again, do not give a positive result in this test. These simple yet effective tests are fundamental in organic chemistry for identifying and distinguishing between aldehydes and ketones in various samples.
Conclusion
Understanding the key differences between aldehydes vs ketones is essential in organic chemistry. Their unique structures and reactivity define their distinct roles in various chemical reactions and applications. Through identification tests like Tollens’ and Fehling’s, and considering their environmental and health implications, we gain a comprehensive view of these important compounds. Whether in industrial applications, biological processes, or everyday products, aldehydes and ketones significantly impact our lives, underscoring the importance of their study and safe handling.