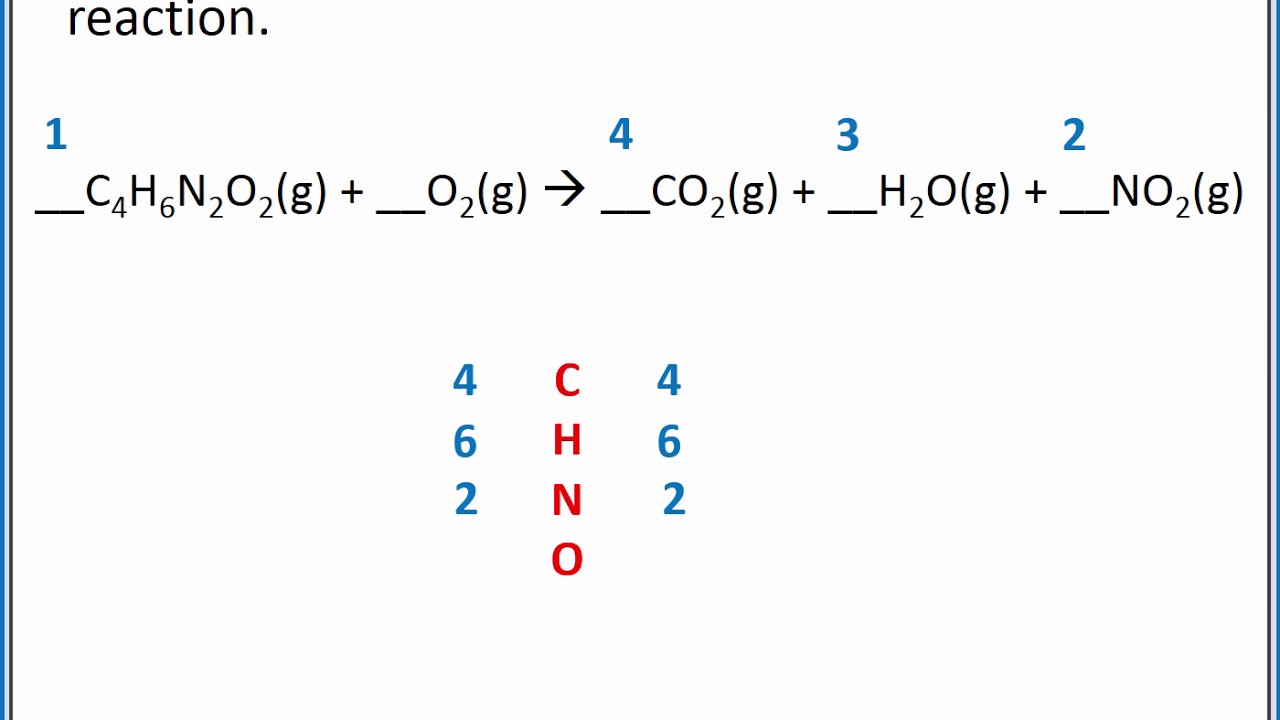
Balancing chemical equations is a fundamental skill in chemistry that ensures the law of conservation of mass is upheld. When you balance a chemical equation, you confirm that the same number of atoms of each element exists on both sides of the equation. This process might seem daunting at first, but with practice and understanding, it becomes straightforward.
Understanding Chemical Equations
Before delving into balancing chemical equations, it’s crucial to understand what a chemical equation represents. A chemical equation depicts a chemical reaction using symbols and formulas. For instance, in the reaction between hydrogen and oxygen to form water, the chemical equation is written as:
H2+O2→H2OH2+O2→H2O
In this equation, H2H2 and O2O2 are the reactants, and H2OH2O is the product.
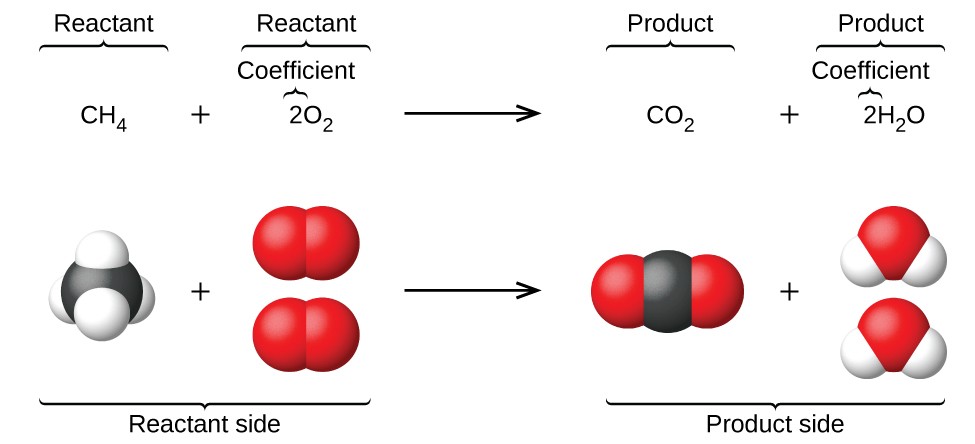
Writing and Balancing Chemical Equations
The Importance of Balancing Equations
Balancing chemical equations is vital because it aligns with the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Consequently, the mass and the number of atoms of each element must remain constant before and after the reaction. This principle ensures that the equation accurately represents the reaction.
Steps to Balance Chemical Equations
To balance chemical equations, follow these steps:
Step 1: Write the Unbalanced Equation
Start with the unbalanced equation, listing the reactants and products. For example:
C3H8+O2→CO2+H2OC3H8+O2→CO2+H2O
Step 2: Count the Atoms of Each Element
Next, count the number of atoms of each element on both sides of the equation. This step helps identify which elements need balancing. In our example, the unbalanced equation has:
- Reactants: 3 Carbon (C), 8 Hydrogen (H), 2 Oxygen (O)
- Products: 1 Carbon (C), 2 Hydrogen (H), 3 Oxygen (O)
Step 3: Balance One Element at a Time
Begin by balancing elements that appear in only one reactant and one product. It’s often easier to start with elements other than hydrogen and oxygen. For instance, balance carbon atoms first:
C3H8+O2→3CO2+H2OC3H8+O2→3CO2+H2O
Now, we have:
Reactants: 3 Carbon (C), 8 Hydrogen (H), 2 Oxygen (O)
Products: 3 Carbon (C), 2 Hydrogen (H), 7 Oxygen (O)
Step 4: Balance Hydrogen and Oxygen Atoms
After balancing carbon, balance hydrogen next. Adjust the coefficient of H2OH2O to balance hydrogen atoms:
C3H8+O2→3CO2+4H2OC3H8+O2→3CO2+4H2O
Now, the counts are:
Reactants: 3 Carbon (C), 8 Hydrogen (H), 2 Oxygen (O)
Products: 3 Carbon (C), 8 Hydrogen (H), 10 Oxygen (O)
Finally, balance the oxygen atoms by adjusting the coefficient of O2O2:
C3H8+5O2→3CO2+4H2OC3H8+5O2→3CO2+4H2O
The balanced equation now reads:
Reactants: 3 Carbon (C), 8 Hydrogen (H), 10 Oxygen (O)
Products: 3 Carbon (C), 8 Hydrogen (H), 10 Oxygen (O)
Checking Your Work
After balancing the equation, verify that the number of atoms for each element is the same on both sides. This confirmation ensures the equation adheres to the law of conservation of mass.
Practice Makes Perfect
Balancing chemical equations requires practice. Start with simple equations and gradually move to more complex ones. Utilize online tools and resources to test your skills and reinforce your understanding.
Conclusion
Balancing chemical equations is an essential skill for any chemistry student. By understanding the steps and practicing regularly, you can master the process. Remember, the key is to ensure that the number of atoms for each element is equal on both sides of the equation. With this guide, you have a solid foundation to begin balancing chemical equations confidently.